HIV infection: pitavastatin dec cardiac events
the recent REPRIEVE trial found that the use of the new statin
pitavastatin was very effective in decreasing cardiovascular events in those
with controlled HIV infection and otherwise very low cardiovasc risk, as well
as documenting its safety (see hiv pitavastatin dec cardiovasc dz NEJM2023 in
dropbox, orDOI: 10.1056/NEJMoa2304146)
Details:
-- 7769 patients aged 40 to 75 and with HIV infection on a stable
antiretroviral therapy and a low-to-moderate risk of cardiovascular disease
based on the 2013 AHA/ACC risk calculator were randomized to pitavastatin 4mg
versus placebo
-- median age 50, 65% nonwhite, 31% women; international study
with 53% from high-income countries, 18% from Latin America/Caribbean, 15%
sub-Saharan Africa, rest from Southeast/East/South Asia
-- median CD4 count was 621 and the HIV viral load was below
quantification (<20 copies/mL) in 88% of the participants and <400 in 98% (no comment on the HIV meds used)
-- median screening LDL was 108 mg/dL (interquartile range
87 to 128); median 10-year atherosclerotic cardiovascular disease risk was
4.5% (low risk defined as <5%) [ie these were really low risk
individuals from a cardiovascular event]
-- primary outcome: the occurrence of major adverse cardiovascular
events (MACE), a composite of cardiovascular death, myocardial infarction,
hospitalization for unstable angina, stroke, TIA, peripheral arterial disease,
revascularization, or death from undetermined cause
-- key secondary outcomes included individual components of the
primary outcome, as well as death from any cause, LDL and non-HDL cholesterol
levels; also assessing targeted safety events (including incident diabetes
mellitus, liver injury, myalgia/muscle weakness, or treatment-limiting
disability)
-- This trial was designed for 8 years but was stopped early at
5.1 years because of clear efficacy of the pitavastatin (rather unusually,
there was no comment in this paper about the anticipated length of the study; I
could only find it on an internet search)
Results:
-- LDL decreased from a median of 107 to 74 mg/dL in the
pitavastatin group, and 106 to 105 mg/dL in the placebo group, and non-HDL
decreased from 133 to 97 mg/dL without change in the placebo group
-- the effects of pitavastatin on LDL and non-HDL
were durable throughout the follow-up.
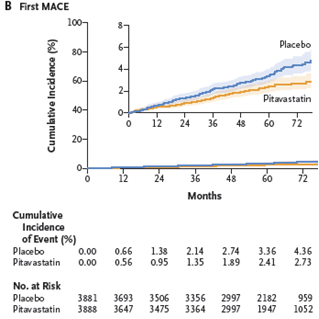
-- major adverse cardiovascular events:
-- 4.81
per 1000 person-years in those on pitavastatin, versus 7.32 per 1000
person-years in the placebo group
-- 35% decrease with medication, HR 0.65 (0.48-0.90), p=0.002
-- this all translates into a number needed to treat (NNT) of 106
patients (64-303) for five years with pitavastatin [this NNT is in the same
range as for treating hypertension]
-- and,
the NNT for patients at higher cardiovascular risk at baseline would be
significantly less than 106
-- individual components of MACE (the most common ones being
cardiac ischemia and stroke or TIA):
-- first
cardiac ischemia or myocardial infarction (80% were type 1): 1.40 per 1000
person-years in pitavastatin and 2.51 in placebo, HR 0.56 (0.34-0.90), a 44%
decrease
-- none
of the other individual components were statistically significant, though all
showed a trend to superiority with pitavastatin (though many of these other
outcomes were uncommon, making statistical significance more difficult to
achieve)
-- no real difference in results in per-protocol analysis
-- no real difference in outcomes for women versus men
-- efficacy of pitavastatin was independent of the global location
of the study, and in both low-income and high-income countries
-- Here’s the graph over time to the first major adverse
cardiovascular event, finding that the curves diverged at about the 12-month
mark and continued to splay apart:
-- Adverse effects:
-- no
difference in total number of nonfatal serious adverse events, 695 on
pitavastatin and 694 on placebo
--
muscle-related symptoms: 91 participants (2.3%) taking pitavastatin and 53
(1.4%) on placebo, incidence rate ratio IRR 1.74 (1.24-2.45)
-- muscle-related symptoms led to withdrawal in 44 participants on
pitavastatin (1.1%) versus 21 (0.5%) on placebo
--
diabetes occurred in 206 participants (5.3%) taking pitavastatin versus 155
(4.0%) on placebo, IRR 1.35 (1.09-1.66)
-- no
significant difference in clinically important rhabdomyolysis, or increased ALT
levels
--
trial discontinuation rates were below the predetermined
thresholds
Commentary:
-- it is well documented that the risk of cardiovascular disease
is increased in patients with HIV infection, even if well-controlled: the risk
in prior studies suggests that HIV confers twice the risk, likely because of
residual inflammation and immune activation/dysfunction, and this seems to be
true after controlling for traditional cardiovasc risk factors
--
however, effective antiretroviral therapy does decrease the cardiovascular
risk, but incompletely
-- this was a drug company sponsored study. the paper comments
that pitavastatin was chosen as the statin because of its lack of interaction
with HIV medications. However, there really are several other statins that also
did not interact, eg neither
atorvastatin nor rosuvastatin interact with Biktarvy or Dovato (though best to
do a drug interaction check on https://www.hiv-druginteractions.org/
). So, I suspect the reason for choosing pitavastatin was a tad self-serving by
the drug company…
-- this trial found that there was a major reduction of clinical
cardiovascular events in a group of people with a quite low 10-year calculated
atherosclerotic risk, in younger patients, and those with a low baseline LDL
level.
-- The
level of cardiovascular protection found in this study was more than what was
predicted by the change in LDL levels alone, as anticipated from other statin
studies with higher initial LDL levels
-- as is well known,
statins do have an array of pleiotropic effects besides lowering LDL, including
decreasing inflammation, oxidative stress, decreased platelet aggregation,
decreased cardiac hypertrophy and fibrosis, improving endothelial dysfunction,
decreasing thrombosis
-- and the benefit of
pitavastatin, as with other statins, is evident within 12 months
-- the
level of protection in this study was similar to that in the JUPITER study (https://www.nejm.org/doi/full/10.1056/NEJMoa0807646
), which similarly enrolled people with baseline lower LDL levels (median 108)
but in that study with inflammation as measured by CRP levels
(high-sensitivity CRP 4.1); they found that rosuvastatin 20mg was associated
with 50% decreases in cardiovascular events after only 1.9 years likely
reflecting the importance of statin-associated decreases in these pleiotropic
effects on clinical outcomes ( anti-inflammatory effects, etc). By the
way, a Mediterranean diet also decreases inflammation, as found in several dietary
studies: eg see https://www.mdpi.com/2227-9059/8/7/201
)
-- as per prior blogs, there are a few issues around LDL levels:
-- LDL
seems to be a surrogate marker for cardiovascular disease, with major
differences between different LDL moieties that electrophoretically migrate in
the LDL range: small, dense LDL particles are three times more atherogenic than
the larger particles, yet both may have the same LDL level: http://gmodestmedblogs.blogspot.com/2023/05/cad-presumed-mechanism-and-apob-was.html
-- for
high-risk people, very low LDLs down to 20 mg/dL seem to confer increased
benefit without harm: http://gmodestmedblogs.blogspot.com/2018/08/very-low-ldl-levels-benefit-without-harm.html
Per a detailed review: the Cochrane collective compared
pitavastatin to other statins for its effect to reduce LDL cholesterol, and
pitavastatin is about 6-fold more potent than atorvastatin, 1.7-fold more
potent than rosuvastatin (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7387421/pdf/CD012735.pdf
). The benefit of pitavastatin was a
bit greater in women than men (LDL decreased by 45.08 vs 39.50 mg/dL for the 4 mg pitavastatin dose). and there were very few adverse effects
leading to dropping out of studies: 3 of 262 people, vs 0 of 109 in the placebo
groups (there were not enough data to assess the risk of withdrawals)
-- pitavastatin is also lipophilic (as is atorvastatin and simvastatin,
but not rosuvastatin) and could have the benefit of decreasing
neuroinflammation and perhaps subsequent development of dementia. But clinical
studies to date have not been impressive for this anticipated result. For
example, a recent systematic review found that there was a 20-30% decreased
risk of dementia and of Alzheimer’s in those on statins, more so if higher
statin doses, and this was independent of patient sex or the lipophilicity of
the statin (eg see statin
dementia decrease EuropSocCardiol2022 in dropbox, or
doi:10.1093/eurjpc/zwab208)
-- the association of pitavastatin and diabetes as well as
myalgias has been found in many statin studies. There are, however, some
questions about the real issues here: see https://gmodestmedblogs.blogspot.com/2017/11/statin-use-and-diabetes-is-that-real.html .
and the benefit of statins seems to outweigh the risks of developing diabetes
anyway; the association with myalgias has been largely debunked in several
studies: http://gmodestmedblogs.blogspot.com/2022/09/uspstf-recommendations-statins-in.html
Limitations:
-- as pointed out in many of the cardiovascular studies, the
combination of multiple cardiovascular outcomes into a single composite entity
is beneficial in determining statistical significance (i.e., lots of patients
with this composite), though this amalgamation is not really so clinically
useful: there is a large difference in the actual personal value between having
a cardiovascular death or a stroke versus a revascularization or
hospitalization for unstable angina (I, for one, would really prefer one of the
latter outcomes...)
-- a trial that is stopped earlier than its expected endpoint does
raise the issue to me of an unequal balance between benefits and risks: the
benefits were clearly shown at a shorter time than the study was designed for, though
longer-term risks may not be identified in that eclipsed timeframe
-- this study included people aged 40 to 75, so likely but unclear
if the benefit of pitavastatin would extend to other age groups
-- it would be important to know exactly what overall
medications these participants took (some meds could increase cardiovascular
disease, eg diabetics on insulin or sulfonylureas), or the HIV meds themselves
(eg abacavir, which is a component of Triumeq, one the new powerful HIV meds;
and protease inhibitors)
-- it is difficult to disentangle all of the potential
contributors to cardiovascular risk in persons with HIV. There may well be
other issues that might affect cardiovascular risk, such as stress, depression,
living situations, socioeconomic status, etc, which might be higher in people
with HIV
So,
-- this study showed an impressive reduction of cardiovascular
events in patients on the new statin pitavastatin. And this occurred in younger
persons and those who had quite low traditional risk for cardiovascular
disease, and began after only 12 months on the med
-- recommendations for HIV patients who are at high cardiovascular
risk does include using a statin; this study extends this benefit to younger
patients at much lower cardiovascular risk
-- it is a reasonable assumption that other statins achieving a
similar degree of LDL reduction would yield similar results; and the
pleiotropic effects of pitavastatin apply to other statins
-- and, of course, primary prevention therapy for those with HIV
should be similar for all patients, emphasizing nonpharmacologic management,
especially diet and exercise
-- I personally have never prescribed pitavastatin so far because
of its being a new medication (and, we have very effective older statins with
known risk profiles) as well as the exorbitant cost of a new medication still
on patent
--
hopefully the prohibitive cost of pitavastatin (90 days of a 1 mg dose being
over $1000) is likely to decrease at some point in the not so distant future,
as its patent expires on February 2, 2024 . and it will be a useful addition
to our statins, since it lowers LDL more powerfully
-- this
study, as well as the Cochrane report noted above, do allay my concerns about
adverse effects of the med
geoff
-----------------------------------
If you would like to be on the regular email list
for upcoming blogs, please contact me at gmodest@bidmc.harvard.edu
to get access to all of the blogs:
go
to http://gmodestmedblogs.blogspot.com/ to see the blogs in reverse chronological order
-- click on 3 parallel lines top left, if you want to see blogs by category,
then click on "labels" and choose a category
-- or you can just click on the magnifying glass on top right, then type in a
name in the search box and get all the blogs with that name in them
if you would like to see the articles in this
blog, please email me.
please feel free to circulate this to others.
also, if you send me their emails (gmodest@bidmc.harvard.edu), i
can add them to the list

Comments
Post a Comment
if you would like to receive the near-daily emails regularly, please email me at gmodest@uphams.org